Macrogen Biobank
Securely Collecting and Managing Valuable Human Bio-Resources for Researchers.
The Macrogen Biobank ensures the safe preservation of human-derived materials and offers valuable information to support groundbreaking medical research.
What is Macrogen Biobank?
What is Macrogen Biobank? Macrogen Biobank is the first human biobank established by a non-medical institution in South Korea, officially approved by the Korea Disease Control and Prevention Agency(KDCA) in May 2024. We systematically collect and securely store
human-derived materials and related data. Through donor consent and deposit procedures, we gather and preserve resources such as blood, urine, tissues, and cells, providing them to researchers for various healthcare studies including disease cause investigation, new
drug development, and medical device innovation.
By collaborating with GenTok, a genetics and microbiome-based health management platform, Macrogen Biobank selectively collects and stores leftover samples from Direct-To-Consumer (DTC) genetic tests. This ensures that clinical samples are provided promptly to
researchers, supports innovation in biotechnology and digital health, and helps accelerate personalized medicine research.
The Macrogen Biobank is committed to empowering Korean researchers with unparalleled access to human biological resources. Key eligibility criteria include:
- Applicants must be Korean researchers residing in Korea.
- Principal investigators must have research projects approved by an Institutional Review Board (IRB)
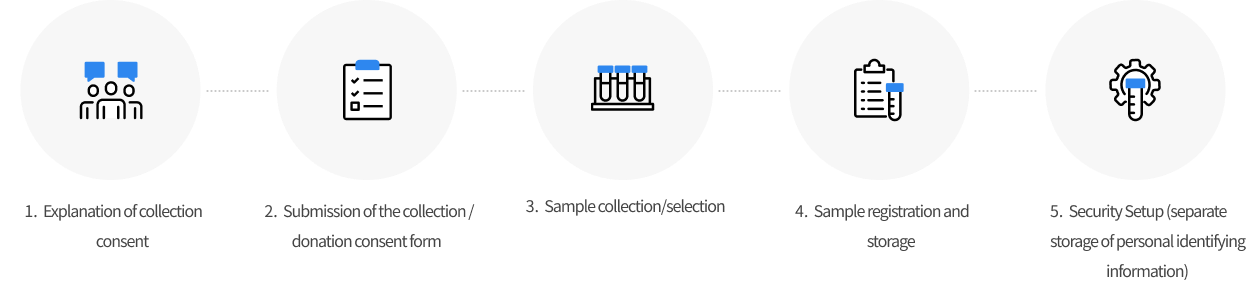
Human Bioresource Collection Process
Pursuant to Article 42 of the Bioethics and Safety Act and Article 40 of its Enforcement Rules, the collection and provision of human bioresources require the donor's written consent.
Any bioresources for which the donor has not provided consent cannot be used for research purposes.
Key Guidelines
- Written Consent Requirement: The collection and use of human biological resources for research purposes are only permissible with the donor's written consent.
- Prohibition Without Consent: Human biological resources cannot be used for research without the donor's explicit authorization.
Once Written Consent Is Obtained
- The leftover samples (e.g., saliva, blood, tissues) generated during testing or research participation are collected and stored by our Biobank.
- Donated human bioresources are used for a range of research purposes, including disease cause identification, new treatment development, and personalized precision medicine studies.
- All collected specimens are anonymized, encrypted, and securely managed in compliance with legal and ethical standards.
Donated human bioresources contribute to public health and scientific advancement, and can also benefit the health of the donor and their family.
Donors may withdraw consent at any time, upon which all collected samples will be immediately descarded to respect the donor's decision.
Collection Process
Human Bioresource Distribution Process
We provide optimal human bioresources to researchers to enhance the quality of research.
-
1Eligibility to ApplyOnly the Principal Investigator (PI) may apply for the distribution of human bioresources.
-
2Required DocumentsA research proposal detailing the purpose and necessity of the study, along with Instituional Review Board (IRB) approval documents, must be submitted.
-
3Review ProcessWe review the submitted documents to determine whether distribution is feasible, and may request additional materials if necessary.
-
4Utilization of Human BioresourcesHuman bioresources provided for research purposes may only be used within the scope specified in the research proposal.
-
5Usage PeriodUse of bioresources is limited to one year from the IRB approval date. If an extension is required, a research amendment request form must be submitted.
-
6Acknowledgment in Research ResultsAny research outputs derived from human bioresources provided by Macrogen Biobank must include an acknowledgment indicating the source of those bioresources.
Distribution Process
